Stress corrosion cracking (SCC) is a mechanical-chemical process leading to the cracking of certain alloys at stresses below their tensile strength
The required elements are a susceptible alloy, the proper chemical environment, and an enduring tensile stress. Usually there is an induction period, during which cracking nucleates at a microscopic level, followed by actual propagation.
SCC is an anodic process, a fact that can be verified by the applicability of cathodic protection (CP) as an effective remedial measure. SCC can sometimes lead to fatigue, or vice versa. Usually, the true nature of the cracking can be identified by the morphology of the cracks. Typically, SCC occurs under only very mildly corrosive conditions, although there are exceptions, as in the cracking of UNS N04400 in aerated hydrogen fluoride (HF) vapors.
There are two distinct types of SCC that affect pipeline steels in soils: high-pH SCC and near-neutral SCC. Table 1 summarises a comparison of guidelines for relevant factors.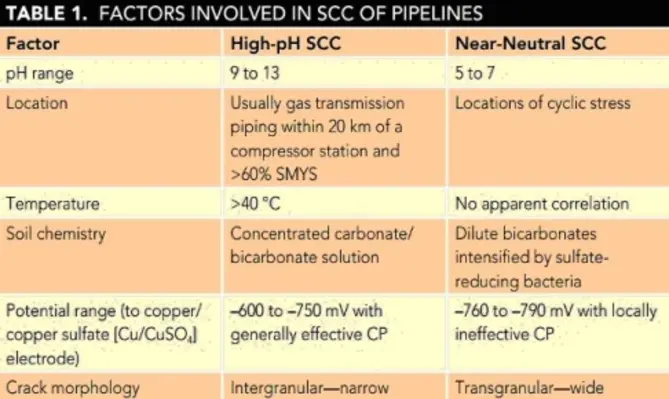
Because the other factors are essentially fixed at susceptible locations, the primary approach to the control of SCC is the maintenance of potentials outside the specified ranges.
There is usually little metal loss or general corrosion associated with a failure by SCC. If there is severe general corrosion, SCC usually will not occur. Thus, the failure of a stressed bolt rusted away until it eventually cannot sustain the applied load is not classified as SCC but as the result of a simple stress corrosion cell. However, if products from general corrosion are trapped so as to exert stress in a structure, they can cause SCC.
While the traditional belief that only alloys and not pure metals are susceptible to SCC may be correct, truly pure metals have few practical applications. Cracking has been observed in materials that would be considered commercially pure, such as copper containing 0.004 per cent phosphorous or 0.01 per cent antimony in environments containing ammonia (NH3) or ammonium ions; steel containing <0.01 per cent carbon along with small amounts of manganese, sulfur, and silicon in a boiling ammonium nitrate solution; or commercial titanium containing 600 ppm of oxygen and 100 ppm of hydrogen.
Table 2 lists some environments in which SCC has been observed for at least some alloys of the systems listed. This listing does not imply that all alloys of a given material will be equally susceptible or that there are none in the class that may be immune to the environments listed.
This article is adapted by MP Technical Editor Norm Moriber from Corrosion Basics: An Introduction, Second Edition, Pierre R. Roberge. It originally appeared in Materials Performance Magazine, www.materialsperformance.com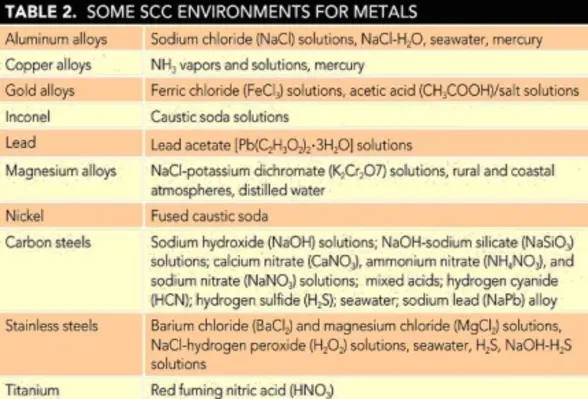
About NACE International
NACE International, The Worldwide Corrosion Authority, was established in 1943 by eleven corrosion engineers from the pipeline industry as the “National Association of Corrosion Engineers.”
Today, NACE serves nearly 36,000 members in over 130 countries and is recognised globally as the premier authority for corrosion control solutions. The organisation offers technical training and certification programs, conferences, industry standards, reports, publications, technical journals, government relations activities and more.
NACE International is headquartered in Houston, Texas, with offices in San Diego, California; Kuala Lumpur, Malaysia; Shanghai, China, Sao Paulo, Brazil and Al-Khobar, Saudi Arabia.
Materials Performance (MP) magazine is the flagship publication of NACE International. Published monthly, MP is the world's largest circulation journal dedicated exclusively to corrosion prevention and control.








