TGS, the global geoscience data provider for oil and gas exploration and production companies, has partnered with Schlumberger for a new 2D seismic project offshore Egypt
Exploration & Production
Eni enters into new exploration permits offshore Morocco
Italian oil and gas giant Eni has signed a petroleum agreement (PA) with the Moroccan State Company ONHYM to enter into the Tarfaya Offshore Shallow exploration permits I-XII, which is located in the Atlantic Ocean offshore the cities of Sidi Ifni, Tan Tan and Tarfaya
Schlumberger wins Aramco contracts for oil and gas well services
Saudi Aramco has awarded two contracts to Schlumberger for drilling rigs and services for oil and gas wells in the Kingdom
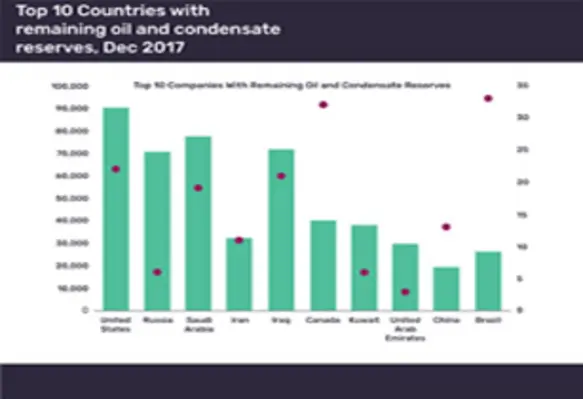
Five Middle East countries in top ten with largest oil reserves: GlobalData
Saudi Arabia, Iraq, Kuwait, Iran and the UAE rank in the list of the ten countries with the largest remaining crude and condensate reserves, according to GlobalData
World Bank to stop funding oil and gas projects from 2019
The World Bank group has announced that it will cease to finance new upstream oil and gas exploration projects after 2019